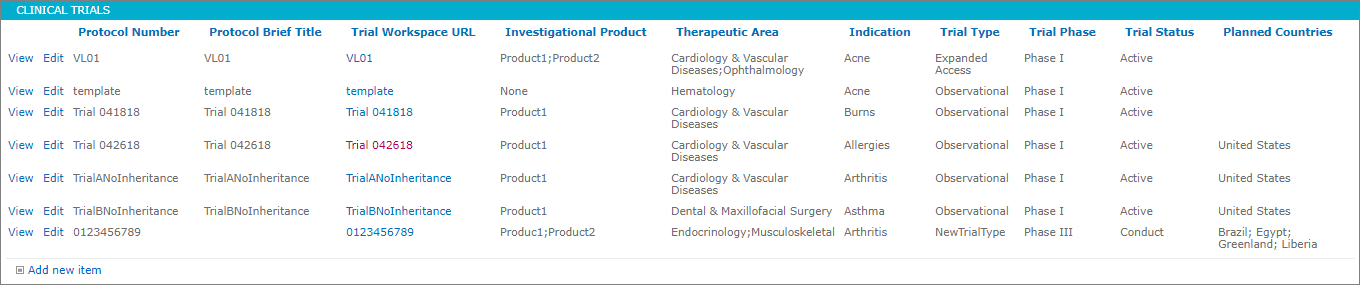
Add a New Clinical Trial
For each clinical trial added, the system creates a workspace. The system allows the user to select a template on which to base the workspace and will apply the predefined security scheme to the workspace. Additionally, it allows the user to select an EDT template that will automatically add placeholders for the required documents defined in the template to the workspace EDT feature.
CAUTION:
It is recommended that Clinical Trials not be deleted as they will still appear on the Clinical Trials list.
To add a clinical trial, the administrator would:
- Access the hub site.
- Select “Clinical Trials” under Reference Data on the Quick Launch Bar.
- Click on the “Add new item” link.
- Enter a Protocol Number.
- Enter a Protocol Brief Title.
- Select a Workspace Template from the menu.
- Select a Document Index from the menu.
- Select the Essential Document Template from the menu.
- Select an Investigational Product or Product(s).
- Select one or more Therapeutic Areas.
- Select an Indication from the menu.
- Select a Trial Type from the menu.
- Select a Trial Phase from the menu.
- Select a Trial Status from the menu.
- Select one or more Planned Countries.
- Click on the “Save” button.
The clinical trial will be added to the Clinical Trials list. The Trial Workspace URL will not be populated until the system has completed building the clinical trial.